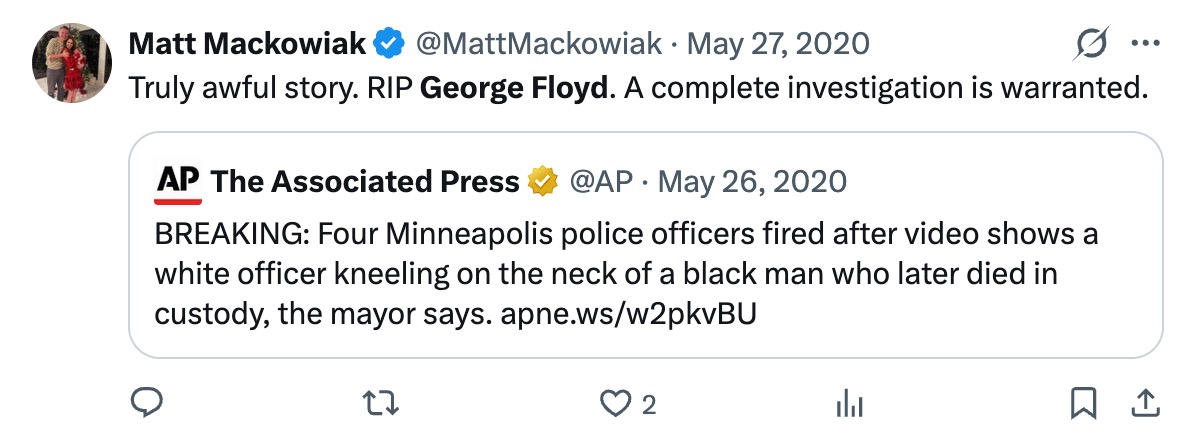❓WHAT HAPPENED: Texas Democrat James Talarico said he loves “trans children.”
👤WHO WAS INVOLVED: James Talarico, the Democrat Texas Senate candidate, and Republican critics, including GOP spokesman Zach Kraft.
📍WHEN & WHERE: Comments made during an August 2023 podcast appearance; Talarico won the Democrat primary in Texas in March 2026.
💬KEY QUOTE: “I love, I’m just saying this because it’s all on my mind, the trans children,” Talarico said.
🎯IMPACT: Talarico’s comments and legislative record have drawn sharp criticism from Republicans, who accuse him of promoting radical transgender ideology.
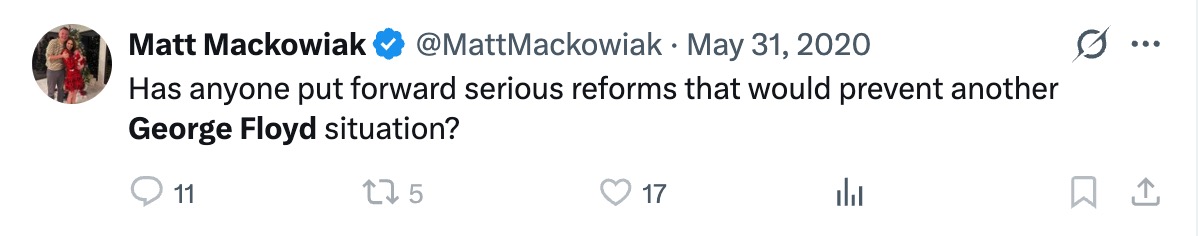
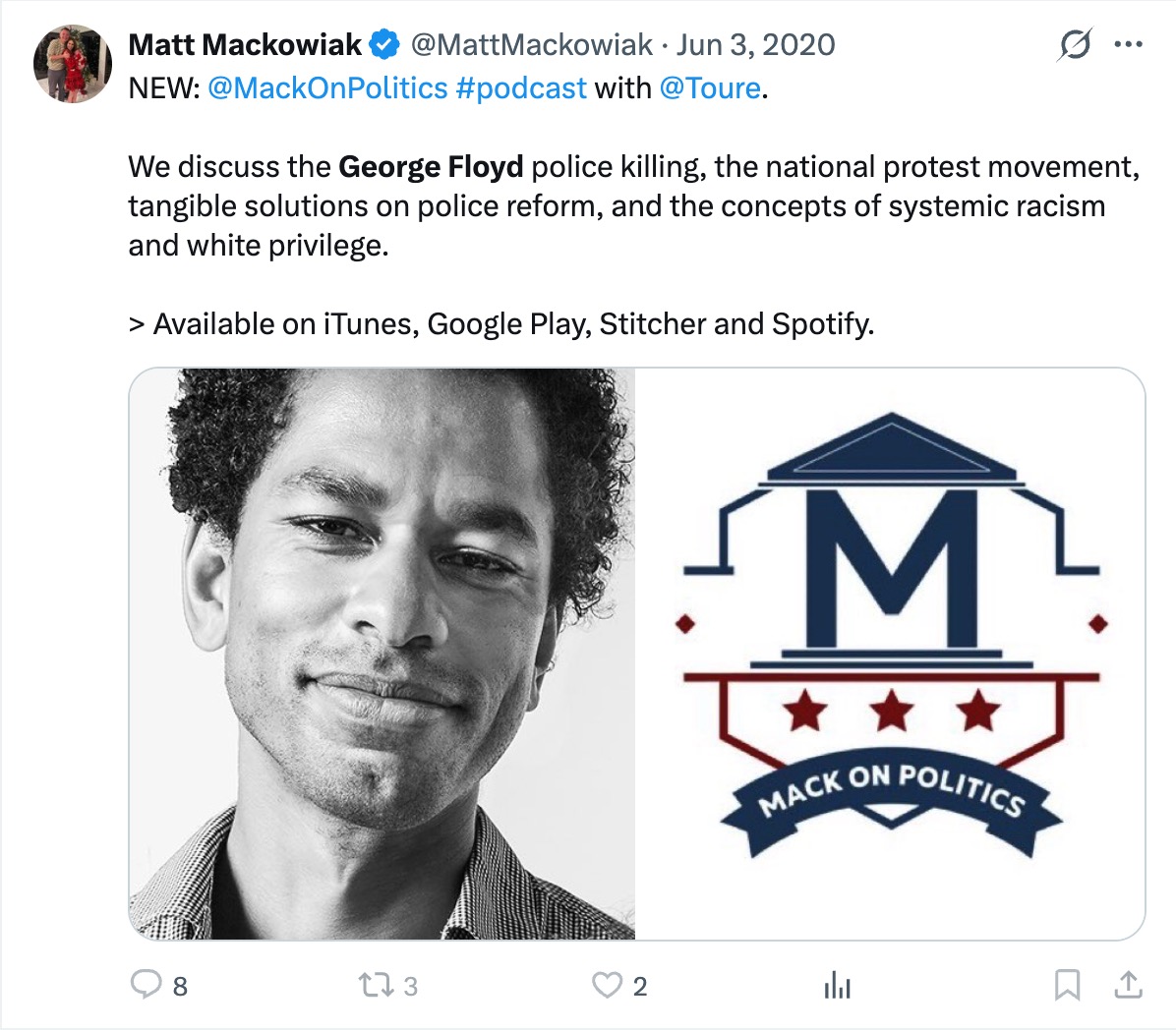
James Talarico, the Democrat nominee for U.S. Senate in Texas, has come under fire for comments made during an August 2023 podcast appearance where he listed “trans children” as something he loves. The remarks, which resurfaced after he won the Democrat primary for a Texas Senate seat, have reignited scrutiny of his legislative record on transgender issues and support for invasive surgeries. In recent months, a number of professional medical associations have reversed their support for transgender procedures for minors, citing severe long-term complications and irreversibility.
“I love, I’m just saying this because it’s all on my mind, the trans children, who showed up yesterday at the state capitol to advocate for their humanity. They shouldn’t have to, but it was an inspiration to watch,” Talarico said during an A Superbloom podcast episode in August 2023. Notably, Talarico has been a longstanding proponent of transgender ideology and allowing minors access to mutilating transgender surgeries and hormone procedures.
Also in 2023, the Texas state Democrat lawmaker opposed a law that prohibited inflicting such practices on minors. Additionally, during a committee hearing, he controversially claimed, “The one thing I want us to all be aware of is that modern science obviously recognizes that there are many more than two biological sexes… In fact, there are six.”
Talarico has also argued that parents who do not “affirm” their child’s gender identity are abusive, claiming it is a “life and death issue.” He has further attacked Republican-backed legislation, including a 2021 bill that barred males from competing in female sports, and has publicly stated that “God is non-binary.”
Join Pulse+ to comment below, and receive exclusive e-mail analyses.