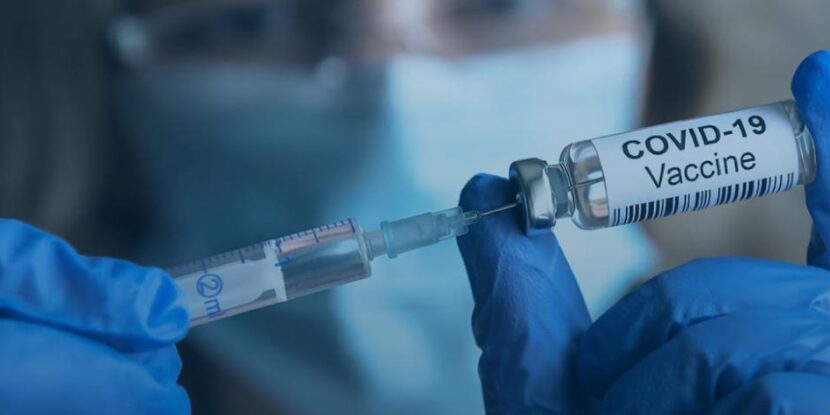
❓WHAT HAPPENED: The U.S. Food and Drug Administration (FDA) approved updated COVID-19 vaccines from Pfizer, Moderna, and Novavax, but limited their use for many Americans, including children under five.
👤WHO WAS INVOLVED: FDA officials, Health Secretary Robert F. Kennedy Jr., and FDA Commissioner Marty Makary.
📍WHEN & WHERE: The decision was announced Wednesday, with vaccines set to ship immediately across the United States.
💬KEY QUOTE: “The American people demanded science, safety, and common sense. This framework delivers all three.” — Robert F. Kennedy Jr.
🎯IMPACT: The updated shots, targeting the LP. 8.1 variant, are approved for seniors and those with at least one high-risk health condition, such as asthma or obesity.
The U.S. Food and Drug Administration (FDA) has approved updated COVID-19 vaccines from Pfizer, Moderna, and Novavax, but with significant limitations for younger populations. The updated shots, targeting the LP. 8.1 variant, are approved for seniors and those with at least one high-risk health condition, such as asthma or obesity. However, access for healthy adults and children has been curtailed, with Pfizer’s vaccine no longer authorized for children under five.
Parents seeking vaccines for young children will need to rely on Moderna’s Spikevax, which is approved for children as young as six months but only for those with serious health conditions. Novavax’s protein-based vaccine, meanwhile, is available for individuals 12 and older under similar restrictions. The FDA’s decision marks a departure from the previous policy recommending vaccines for all Americans six months and older.
Department of Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. and FDA Commissioner Marty Makary spearheaded the policy shift, emphasizing a risk-based approach. “The American people demanded science, safety, and common sense. This framework delivers all three,” Kennedy stated.
Logistical challenges loom as insurers and pharmacists navigate the new framework. Insurers typically follow recommendations from the Centers for Disease Control and Prevention (CDC) advisory panel, which has undergone changes under Kennedy’s leadership. Pharmacists, who administer most vaccines, may face some legal and procedural hurdles in verifying patients’ eligibility under the new guidelines.
The FDA’s decision also revoked emergency use authorizations for Pfizer’s vaccine for young children and other pandemic-era therapies, signaling a shift in the U.S. government’s approach to COVID-19 management.
Join Pulse+ to comment below, and receive exclusive e-mail analyses.