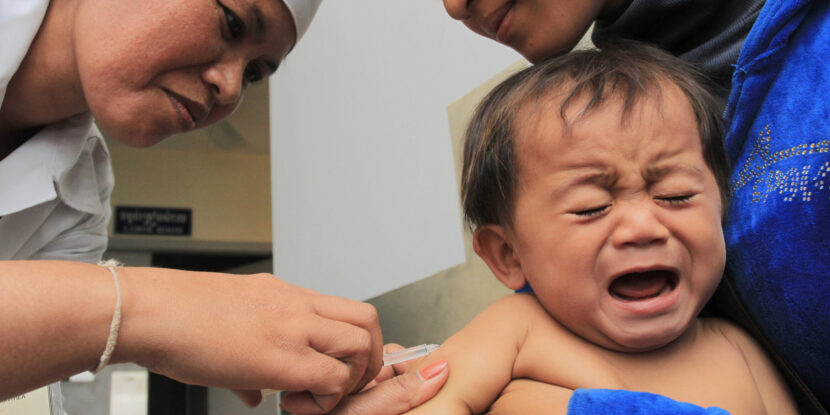
❓WHAT HAPPENED: The U.S. Centers for Disease Control and Prevention (CDC) updated its guidance on hepatitis B vaccines, advising women who test negative for the virus to consult health care providers about vaccinating their newborns within 24 hours of birth.
👤WHO WAS INVOLVED: Acting CDC Director Jim O’Neill, Department of Health and Human Services (HHS) Secretary Robert F. Kennedy Jr., and the CDC’s Advisory Committee on Immunization Practices.
📍WHEN & WHERE: The recommendation was accepted on Tuesday, following a vote earlier this month by the advisory committee, and applies nationwide.
💬KEY QUOTE: “We are restoring the balance of informed consent to parents whose newborns face little risk of contracting hepatitis B.” – Jim O’Neill
🎯IMPACT: The decision has sparked criticism from some lawmakers on Capitol Hill with deep ties to the pharmaceutical industry.
The U.S. Centers for Disease Control and Prevention (CDC) has revised its guidance on hepatitis B vaccinations for newborns. Women who test negative for the virus are now advised to consult with health care providers before deciding whether to administer the first dose of the vaccine to their babies within 24 hours of birth.
The CDC’s Advisory Committee on Immunization Practices proposed the change earlier this month, and Acting CDC Director Jim O’Neill formally approved it on Tuesday. O’Neill explained the decision, stating, “We are restoring the balance of informed consent to parents whose newborns face little risk of contracting hepatitis B.”
Notably, the new guidance still maintains that infants born to mothers who test positive for hepatitis B or whose infection statuses are unknown should receive the vaccine within the first 24 hours of life. For other infants, the CDC now advises delaying the first dose until at least two months of age. Pediatricians are expected to continue recommending the vaccine for newborns, and the Department of Health and Human Services (HHS) has assured that insurance coverage for the shots will remain unchanged.
However, the change is drawing some pushback—especially from lawmakers with deep ties to the pharmaceutical industry. “Ending the recommendation for newborns makes it more likely the number of cases will begin to increase again,” Senator Bill Cassidy (R-LA) complained in a post on X (formerly Twitter) following the announcement of the updated guidance. “This makes America sicker,” he claimed.
Image by Chhor Sokunthea / World Bank.
Join Pulse+ to comment below, and receive exclusive e-mail analyses.