❓WHAT HAPPENED: Texas Attorney General Ken Paxton has filed a lawsuit against the maker of Tylenol over claims the drug can cause autism in young children.
👤WHO WAS INVOLVED: Ken Paxton, Johnson & Johnson, and law firm Keller Postman.
📍WHEN & WHERE: The lawsuit was filed this week in Texas.
💬KEY QUOTE: “Big Pharma betrayed America by profiting off of pain and pushing pills regardless of the risks. These corporations lied for decades, knowingly endangering millions to line their pockets.” – Ken Paxton
🎯IMPACT: The lawsuit marks the first of its kind from a state government and could have significant implications for pharmaceutical companies and consumer safety.
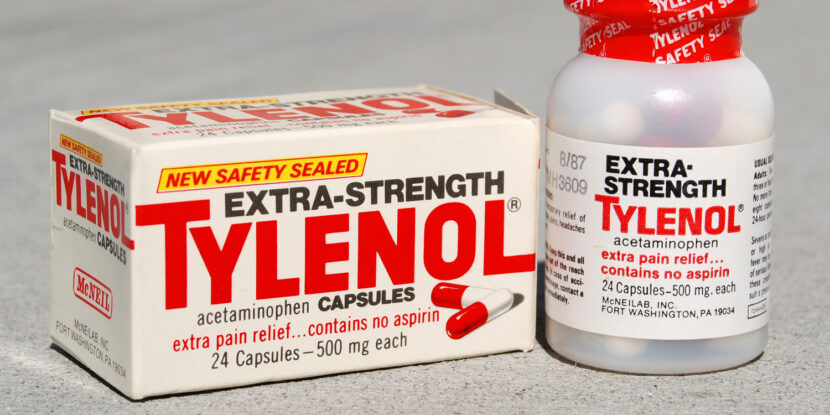
Texas Attorney General Ken Paxton (R) has filed a lawsuit against Johnson & Johnson, the maker of Tylenol, alleging that the company failed to warn consumers that acetaminophen (paracetamol), the drug’s active ingredient, could increase the risk of autism in children when taken during pregnancy. This marks the first time a U.S. state government has brought such a case against the pharmaceutical giant.
Paxton framed the lawsuit as part of his broader effort to hold major drug manufacturers accountable, saying, “Big Pharma betrayed America by profiting off of pain and pushing pills regardless of the risks. These corporations lied for decades, knowingly endangering millions to line their pockets.”
The legal action follows comments from President Donald J. Trump, who recently warned pregnant women against using acetaminophen. “Don’t take Tylenol, don’t take it,” Trump said during a White House press conference, citing concerns about a potential link between prenatal use and autism in children.
The lawsuit, led by the law firm Keller Postman, claims Johnson & Johnson ignored and concealed evidence that acetaminophen could cause neurodevelopmental harm. The firm is also representing individuals in separate cases against the company’s spin-off, Kenvue, over similar allegations.
A 2021 meta-analysis led by researchers from Harvard and Mount Sinai found an increased risk of autism and ADHD among children exposed to the drug in utero. Internal documents obtained through Freedom of Information Act requests have revealed that Food and Drug Administration (FDA) scientists had expressed concerns about acetaminophen’s safety during pregnancy as early as 2016, recommending that the agency urge “judicious” use. Notably, those warnings were never issued publicly.
The FDA has since said it is reviewing labeling updates, but maintains that a causal link between acetaminophen and autism has not been established.
Additional studies cited in the case include research suggesting that prenatal exposure to acetaminophen may disrupt sexual development in male fetuses. Meanwhile, archived guidance from 2017 reportedly showed Tylenol advising pregnant women to avoid all its products, though that warning was later removed.
Join Pulse+ to comment below, and receive exclusive e-mail analyses.