❓WHAT HAPPENED: The U.S. Food and Drug Administration (FDA) has reversed a prior decision from last week and will review Moderna’s flu vaccine for possible approval.
👤WHO WAS INVOLVED: Moderna, the FDA, Dr. Vinay Prasad, and FDA Commissioner Dr. Marty Makary.
📍WHEN & WHERE: February 18, 2026, United States.
🎯IMPACT: The potential approval could make the vaccine available for older adults in the upcoming flu season.
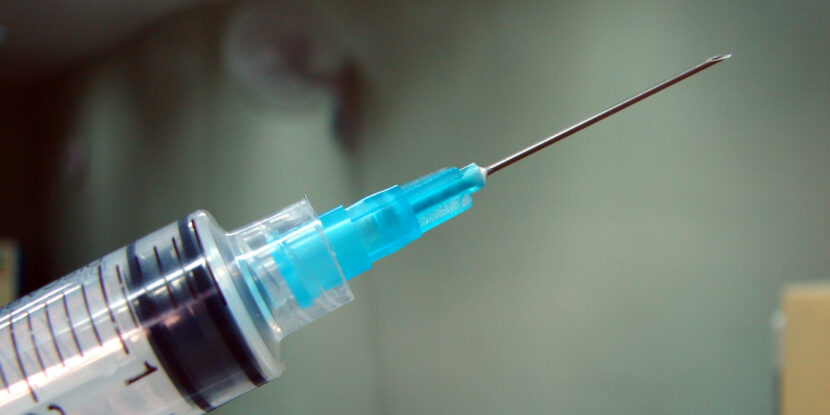
Moderna has announced that the U.S. Food and Drug Administration (FDA) has decided to review its flu vaccine, reversing its previous rejection from last week. The initial rejection cited flaws in Moderna’s research design, but subsequent discussions led the FDA to agree to reconsider. Regulators are now reviewing Moderna‘s vaccine in the United States, Europe, Canada, and Australia for possible commercial sale.
Notably, the company has divided its application by age, seeking traditional approval for individuals aged 50 to 64, and accelerated approval for those 65 and older. An additional study is planned for the older age group once the vaccine is on the market.
Meanwhile, the FDA has set an August deadline to decide on the vaccine’s approval. If approved, it could be available for older adults during next year’s flu season. The vaccine employs mRNA technology, which has been linked to elevated risks of myocarditis and pericarditis, particularly in young males. The National Pulse reported last December that the FDA was considering adding a “black box” warning to COVID-19 vaccines due to the observed risks.
Dr. Vinay Prasad, who initially rejected Moderna’s application, had concerns about the control vaccine used in the study of older adults. Despite this, the FDA is now set to review the application. Moderna’s development of an mRNA flu vaccine is backed by a $750 million investment from Blackstone.
The Wednesday reversal is especially surprising given that FDA Commissioner Dr. Marty Makary addressed the rejection during an event on Tuesday evening, stating, “I think if you’re going to talk about what happened last week, you should know the exact details of the trial results, which are public information.” The remarks—though vague—seemed to allude to Dr. Prasad’s concerns with the study.
Join Pulse+ to comment below, and receive exclusive e-mail analyses.