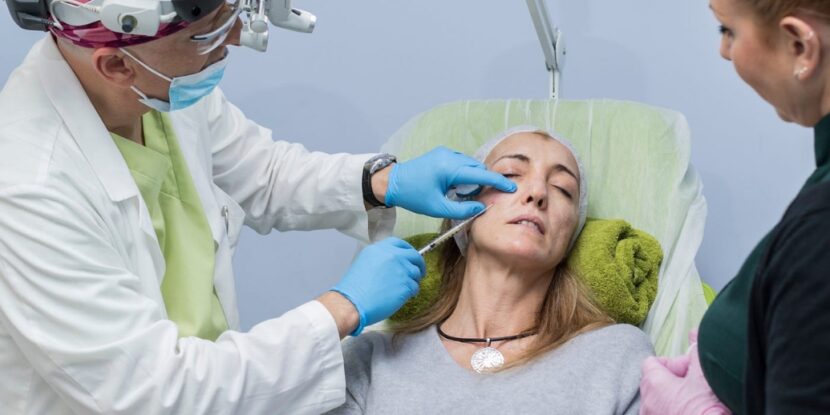
The Centers for Disease Control and Prevention (CDC) confirmed an active investigation into a cluster of illnesses connected to possible counterfeit botulinum toxin injections, commonly known as Botox. The CDC noted its investigation centers around several states and involves injections performed in “nonmedical settings.” These could span homes or cosmetic spas, while the origin of the injections remains “unknown and unverified.”
The Tennessee Department of Health reported four cases treated for botulism-like symptoms after possible counterfeit injections. In Illinois, similar health alarms were raised after two individuals developed symptoms of botulism following Botox injections.
Botulism, though rare, is a serious ailment from toxin interaction with bodily nerves which, if not promptly treated with antitoxins, could result in irreversible damage or death.
While Botox injections do use small doses of toxins to temporarily smooth wrinkles by relaxing facial muscles, approved doses follow stringent Food and Drug Administration guidelines, ensuring purity, sterility, and efficacy, said Dr. Mark Hamilton, a facial plastic surgeon based in Indianapolis.
Shabbir Imber Safdar, executive director of the Partnership for Safe Medicines, a nonprofit coalition advocating against unsafe and counterfeit medicines, stressed the long-term problem of counterfeit injections damaging the health of unaware consumers.
The CDC statement insisted that cosmetic injections should be FDA-approved products, offered by licensed providers in licensed settings. The Plastic Surgery Foundation president, Dr. Scot Glasberg, advised prospective Botox users to adequately assess providers and settings, in light of the associated risks.