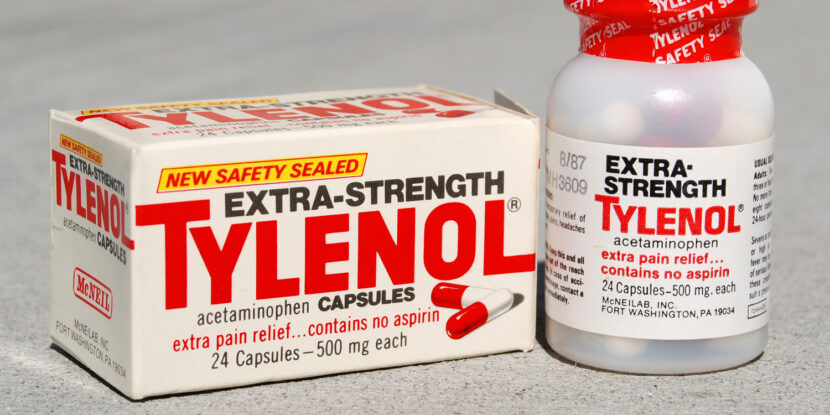
❓WHAT HAPPENED: Internal U.S. Food and Drug Administration (FDA) documents reveal the agency ignored its own safety experts’ advice to warn pregnant women about Tylenol for nearly a decade.
👤WHO WAS INVOLVED: FDA officials, scientists, Tylenol manufacturers, President Donald J. Trump, and Department of Health and Human Services (HHS) Secretary Robert F. Kennedy Jr.
📍WHEN & WHERE: Recommendations were made between 2014 and 2025.
🎯IMPACT: The FDA’s delay in issuing warnings has led to lawsuits, public confusion, and ongoing debates about Tylenol’s safety during pregnancy.
The U.S. Food and Drug Administration (FDA) withheld reports and memos containing repeated recommendations from its own drug safety experts to warn pregnant women about the potential risks of Tylenol, internal agency documents reveal. These recommendations were made across multiple reviews and memos between 2014 and 2022, yet the agency declined to update its guidance until September 2025, following public pressure from President Donald J. Trump and Department of Health and Human Services (HHS) Secretary Robert F. Kennedy Jr.
FDA scientists had reviewed numerous studies showing a potential association between Tylenol use during pregnancy and risks such as ADHD, neurological damage, and other developmental issues. Despite this, FDA leadership repeatedly delayed action, citing insufficient evidence and calling for further studies. A 2016 report by FDA Senior Medical Officer Andrew Mosholder recommended a nuanced warning to pregnant women, but the agency’s leadership postponed decisions, leaving its outdated 2015 statement unchanged for nearly a decade.
The documents obtained by Keller Postman LLC, a law firm pursuing a class-action lawsuit against Tylenol manufacturer Kenvue, show that internal FDA divisions supported issuing warnings as early as 2016. However, FDA officials, including longtime Center for Drug Evaluation and Research (CDER) Director Janet Woodcock, opted against taking immediate action. Woodcock has faced scrutiny for controversial decisions during her tenure, including delays in issuing warnings about other drugs and vaccines.
In November, the Court of Appeals for the Second Circuit will hear an appeal in a class-action lawsuit against Kenvue. The lawsuit alleges that Tylenol manufacturers failed to adequately inform consumers about potential risks, while FDA officials argue that existing evidence does not meet the threshold for causality. The agency’s 2023 review omitted recommendations entirely, further fueling controversy.
The National Pulse reported in September that social media posts by a now-defunct X (formerly Twitter) account belonging to Tylenol recommended multiple times against using acetaminophen, the active ingredient in Tylenol (paracetamol), during pregnancy, due to a potential link to autism.
Join Pulse+ to comment below, and receive exclusive e-mail analyses.